Abstract
Introduction: CSF3R somatic mutations has been included in the 2016 revision of the WHO classification of myeloid neoplasms and acute leukemia as a specific molecular diagnostic marker of CNL. Besides CNL, CSF3R somatic mutation have been reported in about 30% of Severe Congenital Neutropenia (SCN) and in a low frequency of other myeloid malignancies (about 4% of CMML, 1.7% of adult AML, 1.9% of children AML), but not in MDS, JMML and ET (Lavallée Et al., Blood 2016; Sano H et al., Br J Haematol 2015). Lavallée et al. reported for the first time that CSF3R mutation are associated with AML with biallelic mutated CEBPA (4/14, 29%) (Lavallée et al., Blood 2016).We aimed to assess the frequency of CSF3R mutation in MDS, AML and ALL patients and the association with other genetic abnormalities.
Methods: A retrospective analysis of 974 patients with hematological malignancies, including 297 cases of de novo AML, 144 cases of relapsed AML, 268 cases of de novo ALL, 191 cases of relapsed ALL and 74 cases of MDS patients. 58 genes which common mutated in hematological malignancies were tested by amplicon-based NGS with IonTorrent platform (PGM, Life Technology) and the average sequencing depth was 1000x. 36 leukemia related fusion genes were screened by RT-PCR.
Results: We identified CSF3R mutation in 19 patients, which was found in 3.0% (9/297) of de novo AML, 5.6% (8/144) of relapsed AML, 0.5% (1/191) of relapsed ALL and 1.4% (1/74) of MDS. No CSF3R mutation was detected in de novo ALL. All 19 cases had no history of MPN, MDS/MPN or SCN. A total of 8 mutations of CSF3R were detected in 19 patients, 11 cases with T618I mutation, 3 cases with T615A mutation, 1 case with Y779H, Q781*, P809Hfs*8, S810Qfs*6, S813Lfs*2 and D837* mutation respectively, among which 1 patient accompanied with CSF3R T618I and Y779H mutation.
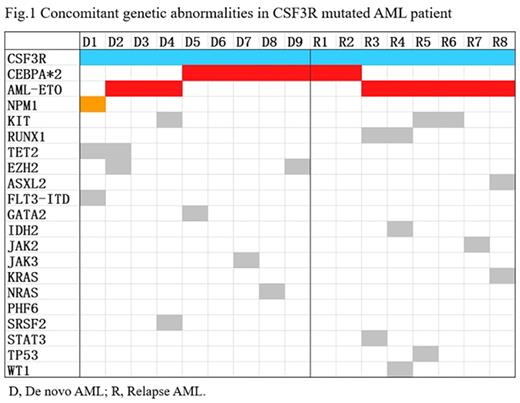
In AML cohort, a total of 17 cases with mutant CSF3R, the median age was 20 years (rang, 6-49) and the frequency of CSF3R mutation have no difference between children and adult. Cytomorphology revealed AML M2 (n=11), AML M4 (n=3), AML M5 (n=1), and 1 case have no data available. The median of CSF3R variant allele frequency (VAF) was 25% (3%-49%), 3 cases with VAF <10%, 10 cases with VAF comparable to the concomitant gene mutation with highest VAF. CSF3R mutation was more common as the predominant clone in relapsed AML (87.5% VS 33.3%, P=0.05). The median of concomitant gene mutations was 2 (range 0-3), double-mutated CEBPA (CEBPAdm) was the most frequently concomitant partner of CSF3R mutation (7/17, 41.2% VS 51/382, 13.4%, P <0.05). We also identified 9 cases with AML1-ETO fusion gene which were mutually exclusive with CEBPAdm. In totally, 16 cases of 17 (94.1%) CSF3R mutated AML patient concomitant with core-binding factor gene abnormalities (Fig.1).
CSF3R mutation have not been reported in ALL, we identified CSF3R mutation in one patient with B-ALL who was 10 years old and relapsed at 43 months after diagnosed. Bone marrow morphology and immunophenotyping at relapse shows no abnormal neutrophils and myeloid lineage expression were found. No fusion genes were detected. CSF3R T618I and ASXL1 Q803* mutations were detected with VAF of 50% and 51%, respectively, which were confirmed of somatic mutation rather than germline origin. As lack of genetic data at diagnosis, we could not analyze the order of mutation acquisition and clone evolution comprehensively. At the time of reporting, the patients have received allo-HSCT for 4 months and acquired sustained remission. We also detected 1 case of MDS patients (MDS-U) carried U2AF1 S34F, CSF3R Q781* and CBL G413S mutations with VAF of 45%, 32% and 31%, respectively.
Due to the limited number of CSF3R mutated cases, we did not perform survival analysis in this study.
Conclusion: (1) CSF3R mutation was found in 3.9% of total AML and 3% of de novo AML cohort, rarely seen in MDS and ALL. (2) In AML, CSF3R mutation are prevalent in M2 and M4, and all concomitant with core-binding factor gene abnormalities (CEBPAdm and AML1-ETO fusion gene) and NPM1 mutation which were associated with favorable-risk prognosis. (3) The CSF3R mutation could appeared as predominant clone or subclone in AML and seemly more common as the predominant clone in relapsed AML. As CSF3R mutation also have implications for therapeutic that JAK inhibitors may be efficacious in treating CSF3R-mutant CNL. The implications of prognostic and target therapy (eg. JAK inhibitors) of CSF3R mutation in AML warranting further investigation.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal